Workflow of imaging mass cytometry
Imaging mass cytometry couples immunohistochemical and immunocytochemical methods with high-resolution laser ablation to CyTOF mass cytometry. Currently, the method allows the simultaneous imaging of 44 proteins and protein modifications at subcellular resolution; with the availability of additional isotopes, measurement of over 100 markers will be possible.
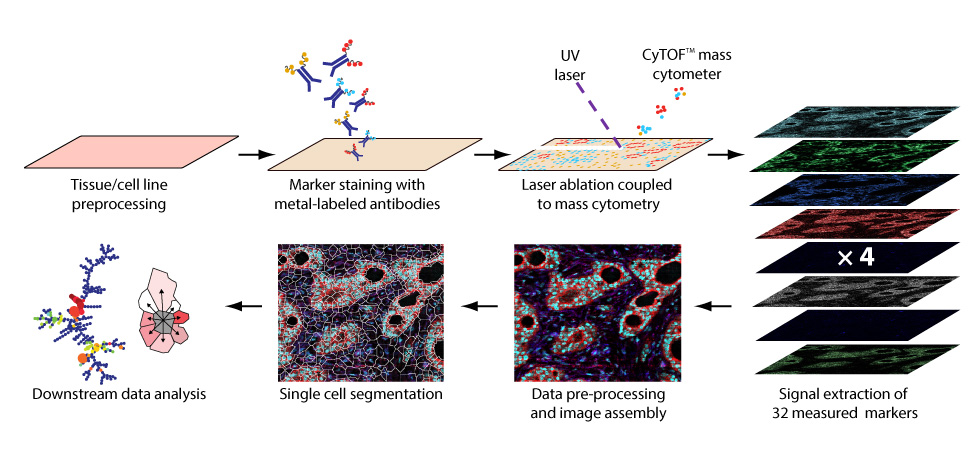
Workflow of imaging mass cytometry (Figure from Giesen et al., Nature Methods, 2014 Apr;11(4):417-22). Tissue sections are prepared for metal-chelated antibody labeling using IHC protocols. Then, tissue samples are positioned in a laser ablation chamber. The tissue is ablated and transported by a gas stream into the CyTOF for mass cytometry analysis. The measured isotope signals are plotted using the coordinates of each single laser shot, and a multidimensional tissue image is generated. Single-cell features and marker expression are determined, allowing the investigation of cell subpopulation properties within the analyzed tissue.
Check out the IMC Forum: Here
Imaging mass cytometry platform

The image shows our current platform to perform the imaging mass cytometry measurements. The pink box to the left is the used laser, the tissue is ablated at the microscope and the tissue particles are measured in the CyTOF2 instrument (orange, to the right).